An orbital is a mathematical function that describes the wave-like behavior of electrons in an atom. For this model the electron under normal conditions always stays at a certain distance from the nucleus and letter people has elaborated that electron location is not fixed though its position could be predicted in where the chance is more to be existed called cloud or electron cloud.

Electron Cloud Model Of Atom Basic Laser Principles Laser It Technologies Quantum Mechanics Quantum Entanglement Quantum Physics
It consisted of a dense nucleus surrounded by a cloud of electrons at various levels in orbitals.

. The modern model is also commonly called the electron cloud model. Thats because each orbital around the nucleus of the atom resembles a fuzzy cloud around the nucleus like the ones shown in the Figure below for a helium atom. What is another name for an electron cloud.
Austrian physicist Erwin Schrödinger 1887-1961 developed an Electron Cloud Model in 1926. Thats because each orbital around the nucleus of the atom resembles a fuzzy cloud around the nucleus like the ones shown in the Figure below for a helium atom. What is another name for the Electron Cloud Model and who is it named after.
The densest area of the cloud is where the electrons have the greatest chances of being. The employers work contract shift created an opportunity for it to help form and transform the workplace. The electron cloud model is known by several different names.
Quantum mechanical model of the atom. What is another name for electron cloud. The cloud is more dense near the nucleus so there is a greater probability that the electron will be near there than anywhere else.
Electron clouds are sometimes referred to as orbitals. Schrödinger and Werner Heisenburg 1901-1976 mathematically determined regions in which electrons would be most likely found. - Another name for this model is Quantum Mechanical - It was named after Erwin Schrodinger.
Helpful 0 Not Helpful 0 Add a Comment. One such example is the Electron Cloud Model proposed by Erwin Schrodinger. With the help of this function the probability of finding an electron in a given.
Taking advantage of key technologies such as cloud solutions mobile platforms and analytics CIOs are well on the mandate to capture and offer valuable insights. Thanks to this model electrons were no longer depicted as particles moving around a central nucleus in a fixed orbit. The electron cloud model uses the concept of orbitals referring to regions in the extra-nuclear space of an atom where electrons are likely to be found.
What is an electron cloud model. Electron clouds are sometimes referred to as orbitals. In the cloud model there are regions where an electron may likely be found but its theoretically possible for it to be located anywhere including inside the nucleus.
The electron cloud model differs from the more simplistic Bohr model in which electrons orbit the nucleus in much the same way as planets orbit the sun. The modern model is also commonly called the electron cloud model.
The Electron Cloud Model Explained Animation Atomic Orbitals Tutorial Crash Chemistry Academy Youtube Chemistry Worksheets Chemistry Help Astrophysics
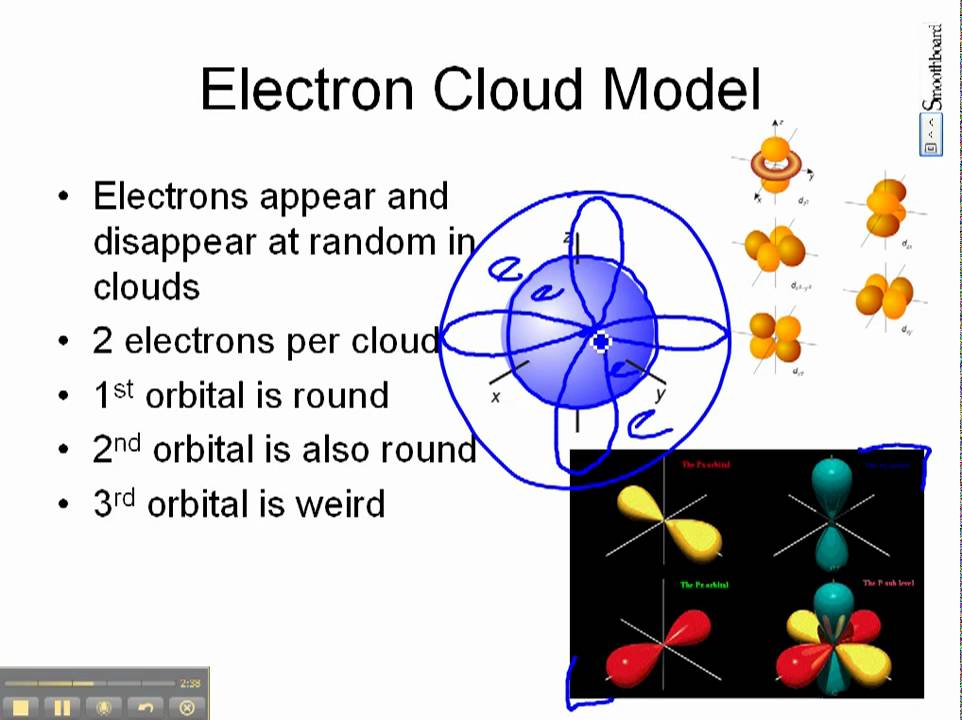
0 Comments